SPL Sorensen,Google Doodle,Google Doodle Is Celebrating S.P.L. Sorensen,S. P. L. Sørensen,Google honors S.P.L. Sørensen, inventor of pH scale, with doodle
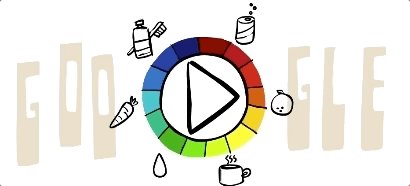
S.P.L. Sorensen Google Doodle (Picture: Google) The Google Doodle continues its tradition of honouring obscure but important figures from history, today celebrating Danish chemist S.P.L. Sorensen. You may not have heard of Søren Peder Lauritz Sørensen, but you will certainly have come across his invention, the pH scale, in chemistry lessons at school. The Dane came up with the scale in 1909 as a way of measuring how acidic or alkaline a substance is and it is still used across the globe today. The Google Doodle today asks you to guess the acidity or alkalinity of various foods and drinks with lower than 7 being acidic and higher being alkaline. S.P.L. Sorensen (Picture: Wikimedia) What is the pH scale? The name of the scale stands for ‘potential of Hydrogen’ and basically measures the concentration of hydrogen ions in a solution. Seven on the pH scale is neutral (blood and water being examples of neutral substances), with lower than seven being acidic and higher than seven described as ‘base’. The scale runs from 0-14 although it is possible to go higher and lower than this in extreme circumstance. Caption: S.P.L. Sorensen Google Doodle (Picture: Google) What does the Google Doodle show us? It is simply the pH levels of the substances pictured, which are as follows… Tomatoes: 4 Eggs: 8 Broccoli: 9 Lemons: 2 Amonia solution: 11 Battery acid: 1 Caption: S.P.L. Sorensen (Picture: Wikimedia) What else do we know about Sørensen? Born in Havrebjerg, Denmark on 9 January 1868, Søren Peder Lauritz was the head of the Carlsberg Laboratory in Copenhagen from 1901 to 1938. He had originally gone to the University of Copenhagen to study Medicine, but switched to chemistry, with clearly exceptional results. The Calrsberg lab had been set up by the famous brewery of the same name to focus on advances in brewing, although Sørensen worked on a range of fields. He was married twice, with his second wife, Margrethe Høyrup, a huge influence on his career as the two worked together at the lab. Sørensen died in February 1939 at the age of 71.
/cdn.vox-cdn.com/uploads/chorus_image/image/59882971/478891.501.0.jpg)
Nearly 110 years ago, while running experiments with beer at the world-renowned Carlsberg research lab in Copenhagen, Danish chemist Søren Peter Lauritz Sørensen developed the simple yet enduring pH scale, which measures whether a substance is acidic or basic. Sørensen’s landmark invention is celebrated in Tuesday’s interactive Google Doodle, which lets you sort sour and bitter foods on different sides of the pH scale to find out how it works.
Many of us already have an intuitive grasp of which side of the scale tomatoes or broccoli fall on thanks to our own built-in pH tester, our tongues. Slightly bitter-tasting foods like leafy greens and legumes have a pH higher than 7, marking them as alkaline, or basic. Sour foods like lemons have a pH lower than 7, making them acidic. Pure water, which is neutral, sits right at 7.
/cdn.vox-cdn.com/uploads/chorus_asset/file/11439951/Screen_Shot_2018_05_29_at_10.29.09_AM.png)
Born to a farming family on January 9, 1868, in a tiny town near the coast of Denmark, Sørensen studied science and started his early career consulting for the Danish navy. He earned his doctorate for his research on cobalt oxalates, complex inorganic structures that have applications in nanotechnology.
At the age of 33, he was appointed as the head of chemistry at the Carlsberg Laboratory in Copenhagen, an institution founded to answer this question: How do you brew the best beer of the highest quality?
The laboratory was already famous for being the first place to cultivate pure yeast and for developing the Kjeldahl method, a technique for measuring the nitrogen content in food and beverages that’s still in use today.
Sørensen soon added more jewels to the lab’s crown. He was primarily studying fermentation, as one does when one works at a lab supported by a brewing company. In particular, he studied the formation of amino acids, the building blocks of proteins.
/cdn.vox-cdn.com/uploads/chorus_asset/file/11440719/SPL_Sorensen.jpg)
He also studied the enzymes made from proteins and quickly realized that hydrogen ion concentrations were important to how to these enzymes performed their functions. He developed the pH scale as a way to keep track of these conditions in a solution.
But what does the scale actually measure?
The term pH means “potential of hydrogen,” and the scale is the negative base 10 logarithm of the concentration of positively charged hydrogen in a solution. Let’s break that down: The concentration of hydrogen ions, a.k.a. protons, in a liquid determines how acidic or basic it is, but this amount can vary drastically, which is why scientists use a logarithmic scale, where each unit changes by a factor of 10. And since the scale is negative, the smaller the number, the more concentrated the protons.
That means that a substance with a pH of 4 is 10 times more acidic than one with a pH of 5 and 100 times more acidic than a pH of 6.
This scale, which runs from 0 to 14, takes a complicated chemical phenomenon and distills it into an easy-to-grasp metric.
It’s now used widely throughout the sciences in applications ranging from designing batteriesto diagnosing blood disorders to measuring humanity’s impact on the ocean, and remains Sørensen’s most famous accomplishment.
The Carlsberg laboratory was Sørensen’s scientific base for the rest of his life. His accomplishments earned him memberships in scientific societies around the world, and his colleagues remembered him as a genial educator. “He was kindly, courteous, ever-willing to listen to those who had not his fund of knowledge and always ready and glad to impart something from his vast store of learning,” wrote A.J. Curtin Cosbie in the journal Nature in an obituary for Sørensen, who died February 12, 1939.
Cosbie also wrote, “Sørensen’s classic work on hydrogen ion concentration will remain as a permanent monument among those who know little of his other work.”
So raise a glass of your favorite drink to toast Sørensen, and perhaps check its pH before you take a sip.
Have you peeped Google today? It’s all about S. P. L. Sørensen, a famed Danish chemist.The search engine site, which sometimes uses its homepage to honor prominent figures, is highlighting the influencer, who introduced the concept of the pH scale.
Born in Denmark in 1868, the scientist gravitated towards medicine at a young age. However, after enrolling at the University of Copenhagen, he shifted gears to chemistry.
In the early 1900s, he rose to fame as the head of the Carlsberg Laboratory. While there, he developed the pH scale. He created the method of measuring acidity and alkalinity while studying ion concentrations.
Introduced in 1909, the system is now universally used for water treatment, medicine and other forms of science. It is also taught in classrooms across the globe.
But Sørensen didn’t stop there. He continued to conduct a variety of other experiments, working at the Carlsberg Laboratory for 37 years. His second wife Margrethe Høyrup Sørensen was one of his closest collaborators.
In 1939, he died at the age of 71, but his contributions to science are still celebrated today by many, including Google.
It’s paying tribute with an doodle, which illustrates the scientist and his pH scale. Viewers who choose to interact must determine where everyday items, such as tomatoes, lemons, eggs and broccoli, are acidic or alkaline.

No comments
Post a Comment